How Sugar, Too Much Protein, Inflammation and Injury Could Drive Epigenetic Cellular Evolution Toward Cancer
It is a commonplace in cancer science to think that cancer cells arise from some kind of cellular evolution, in which cancer cells outcompete normal cells. The standard view is that cancer cells evolve from genetic cellular evolution, but there is a problem with this view: almost all genetic mutations make a cell less fit—that is, less able to compete—in almost every way.
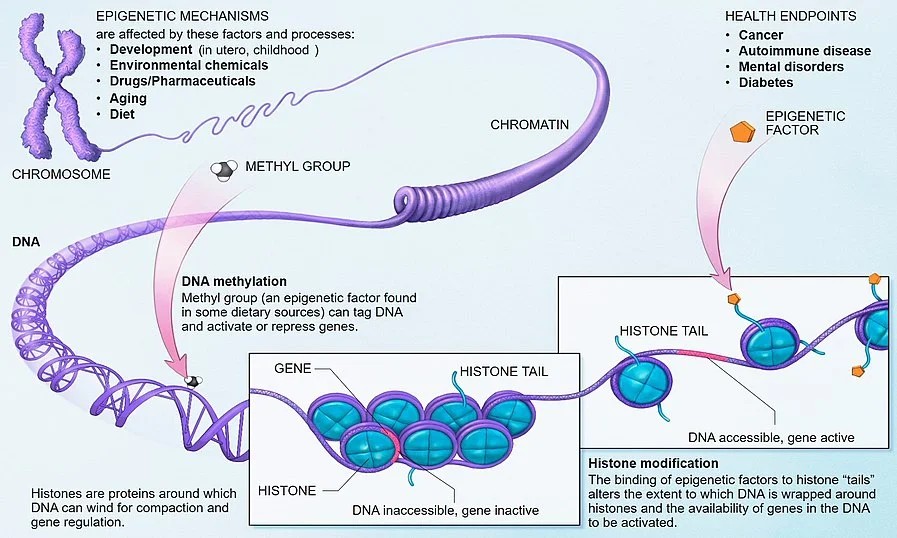
My view—consistent with that of a minority of cancer researchers—is that cancer cells evolve through epigenetic cellular evolution. Epigenetics is the set of switches that tells a cell which to express of all the capabilities already there in its genetic code. Epigenetics is what drives cellular differentiation for normal cells. Other than eggs and sperm, which have only random halves of your genes, all of the normal cells in your body have essentially the same genetic code, but act very differently from each other because of their epigenetic settings. Genetically, every normal cell has the programming for all the capabilities expressed by any cell in your body. Cancer cells typically have some damage that leaves them short of some capabilities (see Good News! Cancer Cells are Metabolically Handicapped), but using the capabilities that remain, they can often get an advantage by having a different set of capabilities switched on than the surrounding normal cells. Under this epigenetic cellular evolution theory of cancer, cellular evolution doesn't have to do the work of hundreds of millions of years of regular evolution, it only has to flip a few switches to turn on capabilities already there from the hundreds of millions of years of genetic evolution behind the normal cells the cancer cells descend from, perhaps a few dozen or a few hundred cell divisions back.
Let me pursue a stark analogy between cancer and ethnic cleansing in Yugoslavia. Through epigenetic evolution, cancer cells turn on a preexisting capability for unbridled cell division and brutality against surrounding cells. This is like the evolution of ideas rationalizing ethnic cleansing turning on a preexisting capability for rape, murder and other forms of brutality in many men who were normal citizens of Yugoslavia a few years earlier.
Many facts about cancer point to some form of evolution. But evolution need not be genetic. Evolution can act on many substrates. The 4th book I feature in "Five Books That Have Changed My Life" is Daniel Dennet's book Darwin's Dangerous Idea:
On page 343, Daniel Dennett emphasizes how general the principles of evolution are:
The outlines of the theory of evolution by natural selection make clear that evolution occurs whenever the following conditions exist:
- variation: there is a continuing abundance of different elements
- heredity or replication: the elements have the capacity to create copies or replicas of themselves
- differential "fitness": the number of copies of an element that are created in a given time varies, depending on interactions between the features of that element and features of the environment in which it persists
All of these conditions are fulfilled for epigenetic switches.
- In addition to regular cellular differentiation, the vicissitudes a cell is subject to and fusion of separate cells into one cell creates epigenetic variation.
- With some imperfection, the epigenetics of a cell tends to be inherited by its daughter cells.
- The effect of epigenetics on growth and cell division, on whether a cell is treated as friend or foe by immune cells, on the susceptibility to signals for self-destruction, the ability to grab key resources, and the ability to travel to new and promising places in the body to set up shop create differential fitness for cells.
Let's do a rundown of the distinguishing characteristics of cancer cells to see why the genetic potential for each of these capabilities would be there already in normal cells, though often not turned on on. For this list, let me quote from Chapter 2 ("Confusion Surrounds the Origin of Cancer") of Thomas Seyfried's book Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. Thomas Seyfried:
In a landmark review on cancer, Drs. Hanahan and Weinberg suggested that six essential alterations in cell physiology were largely responsible for malignant cell growth [5]. This review was later expanded into a book on the Biology of Cancer [31]. These six alterations were described as the hallmarks of nearly all cancers and have guided research in the field for the last decade [32]. The six hallmarks (Fig. 2.2) include the following (italics and punctuation added to the headings):
1. Self-Sufficiency in Growth Signals. This process involves the uncontrolled proliferation of cells owing to self-induced expression of molecular growth factors. Inother words, dysregulated growth would arise through abnormal expression of genes that encode growth factors. The released growth factors would then bind to receptors on the surface of the same cell (autocrine stimulation) or bind to receptors on other nearby tumor cells (paracrine stimulation), thereby locking-in signaling circuits that perpetuate continuous replication. Complicated cybernetic-type diagrams are often presented to illustrate these phenomena (Fig. 2.3). Cybernetics is generally viewed as the study of goal-directed control and communication systems [33]. The abnormal circuitry in tumor cells is assumed to result in large part from the dominant expression of cancer-causing oncogenes.
2. Insensitivity to Growth-Inhibitory (Antigrowth) Signals. In order to carry out specific functions in mature differentiated tissues, most cells must remain quiescent or nonproliferative. A complex signaling circuitry involving the action of tumor-suppressor genes is necessary to maintain the quiescent state. In addition to these internal signals, interactions with other cells (cell–cell) and the external environment (cell–matrix) also act to maintain quiescence. Damage to suppressor genes or the microenvironment is assumed to dampen growth inhibition and provoke proliferation, as the cell no longer responds appropriately to the growth-inhibitory actions of these genes or molecules. Tumor cells are known to express multiple defects in tumor-suppressor genes and in cell–cell or cell–matrix interactions.
3. Evasion of Programmed Cell Death (Apoptosis). Programmed cell death is an effective means of eliminating damaged or dysfunctional cells. Elimination of damaged cells is necessary in order to maintain tissue homeostasis and health. Cell damage can initiate the release of mitochondrial cytochrome c, a protein of the mitochondrial electron transport chain, which is a potent inducer of apoptosis in normal cells. In contrast to normal cells, however, tumor cells lose their sensitivity to apoptotic death signals. Consequently, tumor cells continue to live and proliferate despite damage to their nuclear DNA and respiration. Loss of tumor-suppressor genes, which sense cell damage and initiate cell death, is responsible in part for resistance of tumor cells to programmed cell death. The acquired resistance to apoptosis is a recognized hallmark of most cancers [5, 32].
4. Limitless Replicative Potential. All cells of a given species possess a finite number of divisions before they reach mortality. This is a cell-autonomous program that induces senescence and prevents immortality [5]. Tumor cells, however, lose responsiveness to this program and continue to divide. The phenomenon of limitless replicative potential is closely connected to the first three acquired capabilities.
5. Sustained Vascularity (Angiogenesis). Angiogenesis involves neovascularization or the formation of new blood capillaries from existing blood vessels and is associated with the processes of of tissue inflammation and wound healing. Many solid tumors have difficulty growing unless enervated with blood vessels, which can deliver nutrients while removing metabolic waste products (Fig. 1.3). The dissemination of tumor cells throughout the body is assumed to depend in part on the degree of tumor vascularization. The more blood vessels in tumors, the greater will be the potential to invade and metastasize. Tumor cells release growth factors that stimulate nearby host stromal cells (vascular endothelial cells and macrophages) to proliferate, thus providing the tumor with a vasculature and the means for more rapid growth. The endothelial cells form the vessel walls, while the local macrophages and other stromal cells degrade the microenvironment facilitating neovascularization. A switch from low vascularization to high vascularization is considered to be an essential acquired capability for tumor progression [5, 32, 34].
6. Tissue Invasion and Metastasis. Invasion of tumor cells into local tissue and their spread to distant organs underlies the phenomenon of metastasis. Metastasis or complications of metastasis is associated with about 90% of all cancer deaths [32, 35]. The prevention of metastasis remains the single most important challenge for cancer management.
Growth and Division: The capability for growth and division relevant for characteristics 1, 2 and 4 of cancer cells is definitely something normal cells have to have already in their genes. This means that all it takes to get these characteristics of cancer is to disable the control mechanisms that keep cell growth and division in check. Disabling something—making it not work—is relatively easy. Indeed, disabling is easy enough that it is logically possible that a genetic change rather than an epigenetic change could do it. But direct evidence suggests that, in fact, the control mechanisms that keep cell growth and division in check are disabled epigenetically. In Thomas Seyfried's Chapter 11, "Mitochondria: The Ultimate Tumor Supressor," he gives these remarkable facts:
It is also well documented that nuclei from cancer cells can be reprogrammed to form normal tissues when transplanted into normal cytoplasm despite the continued presence of the tumor-associated genomic defects in the cells of the derived tissues. Dramatic evidence for this fact was obtained from studies in neoplastic tissue from frogs and mice. McKinnell et al. [16] provided some of the first evidence showing that tumor cell nuclei could direct normal vertebrate development following transplantation of the tumor cell nucleus into an enucleated normal egg cell. [From section 11.4]
... nuclear/cytoplasmic hybrids derived by fusion of cytoplasts from malignant cells (nucleus absent) with karyoplasts from normal cells (nucleus present) produced tumors in 97% of the animals injected. These findings showed that normal cell nuclei could not suppress tumorigenesis when placed in tumor cell cytoplasm. In other words, normal nuclear gene expression was unable to suppress malignancy. These findings showed that it was the cytoplasm, rather than the nucleus, that dictated the malignant state of the cells. Although these investigators did not define the molecular basis for the cytoplasmic mediation of tumorigenesis, they suggested that epigenetic alterations of nuclear gene expression might be responsible. [From section 11.2]
Not Committing Cell Suicide: Every normal cell in the body clearly has to have the genetic potential to not kill itself. Again, to get this characteristic 3 of cancer cells requires disabling a self-destruct mechanism, not building anything new.
Being Able to Get New Blood Vessels to Form: Both when a fetus is developing in the womb and in repairing an injury, new tissue needs to be formed. Most types of tissue require nourishment from blood. So most types of normal cells have to have in their fairly immediate cell ancestry cells that had the capability to signal blood vessels—at the least small capillaries—to form within that tissue. Because this capability needs to be switched on again when repairing an injury, injuries are likely to put cells one step closer to being in the cancerous state.
Tissue Invasion and Metastasis. Metastasis is when cancer travels to a distant part of the body rather than just growing to impinge on neighboring parts of the body. There is a subset of normal cells that have to be able to travel all over the body: cells of the immune system. Thomas Seyfried argues at length in Chapter 13, "Metastasis," that a cancerous but not metastatic cell can gain metastatic capabilities for a descendant of sorts by fusing with myeloid cells—immune cells with their origin in the bone marrow—particularly macrophages, a type of white blood cell.
Fusion of a cancerous but not metastatic cell with a macrophage is like a criminal corrupting and teaming up with a trusted police officer. All sorts of things become possible when police credentials can be used to further criminal activity in the one case or white blood cell credentials can be used to further the spread of cancer in the other.
Implications for Cancer Prevention
Avoid Inflammation. A lot is known about risk factors for cancer. Many of these risk factors make more intuitive sense when one realizes that flipping a few epigenetic switches can put a cell on the road to cancer even without any damage to the genes in the cell's nucleus. Many cancer risk factors can be seen as working through causing inflammation. In the first paragraph of Chapter 19, "Cancer Prevention," Thomas Seyfried lays explains the importance of inflammation:
... It is well documented that the incidence of cancer can be significantly reduced by avoiding exposure to those agents or conditions that provoke tissue inflammation, such as smoking, excessive alcohol consumption, carcinogenic chemicals, ionizing radiation, and obesity [2–5].
Elevated levels of inflammation biomarkers (IL-6, IL-8, C-reactive protein, etc.) predict increased risk of cancer [6]. Chronic inflammation, regardless of its origin, damages tissue morphogenetic fields and the epithelial and mesenchymal cells within the field [7–15]. Most importantly, inflammation damages cellular mitochondria, thus reducing the efficiency of OxPhos. Reduced OxPhos efficiency initiates a mitochondrial stress response (RTG signaling) within cells (Chapter 10). RTG signaling is needed to upregulate either glycolysis in the cytoplasm or amino acid fermentation in the mitochondria. Only those cells that can enhance their fermentation in response to respiratory damage will survive. Cells incapable of enhancing fermentation will die from energy failure. As mitochondrial function maintains the differentiated state, cells that upregulate fermentation for survival are at increased risk of becoming less differentiated and ultimately transformed. Prolonged reliance on fermentation destabilizes the nuclear genome, thus initiating the path to carcinogenesis and frank neoplasia. Inflammation damages cellular respiration; damaged respiration is the origin of cancer.
This passage needs some unpacking. A "morphogenetic field" is a pattern of epigenetics in neighboring cells that helps each cell do what it should within a larger tissue or organ. OxPhos is the main generator of ATP energy packets in normal cells. OxPhos depends on delicate structures within mitochondria. Damage to OxPhos puts the affected cells at a metabolic disadvantage to normal cells, as I discussed in "Good News! Cancer Cells are Metabolically Handicapped. But cells with damaged OxPhos are often able to turn on the backup energy generation system of "fermentation." If they can't turn on the backup energy generation system of fermentation, they die. On backup power, cells are not good at keeping their nuclear genes intact when they divide. So even if the key pathway to cancer is epigenetic rather than genetic, the nuclear genes will indeed be messed up in cancer cells.
Note also that nuclear genes can be part of the cause for cancer even if the key pathway involves either inflammation or more direct damage to mitochondria: nuclear genes can make the body more prone to inflammation or nuclear genes can create extra dangers for mitochondria. And of course mitochondrial genes (in the mitochondria outside the nucleus) can make mitochondria extra vulnerable. (Mitochondrial genes are inherited from one's mother.)
Avoid Injury. Above, I discussed how injury is likely to switch on genes for vascularization. Injury also often calls on immune cells that could be in danger of corruption by fusion with cancerous or precancerous cells. Finally, injury causes inflammation.
Starve Cancer Cells. I pursued the logic of starving cancer cells in "How Fasting Can Starve Cancer Cells, While Leaving Normal Cells Unharmed." Fermentation allows cancer cells to metabolize sugar and protein—most directly the amino acid glutamine—but according to Thomas Seyfried, cancer cells are not good at metabolizing fat. From Section 15.2, "Tumor Cell Fitness in Light of the Evolutionary Theory of Rick Potts," Thomas Seyfried writes:
Ketone bodies and fats are nonfermentable fuels in mammalian cells. Tumor cells have difficulty in using ketone bodies and fats for fuel when glucose is reduced. Because tumor cells lack genomic stability, they are less able than normal cells to adapt to changes in the metabolic environment.
Increasing or decreasing the availability of glucose and glutamine, or the availability of ketone bodies and fats to cells of all types should affect the fitness of cancerous or precancerous cells relative to normal cells. If glucose and glutamine are the most nutritious food for cancer cells, that matters for epigenetic evolution. Here are some key practical points:
- Easily digestible carbs make glucose more available to cells.
- Fasting makes glucose and glutamine less available and fats and ketone bodies more available to cells.
- Less is known about which foods make glutamine especially easily available to cells once the body has begun processing those foods. But let me put out there the testable hypothesis that meat and milk cause a bigger spike in glutamine availability to cells than an equal amount of protein in vegetables—not because there is less glutamine in vegetables, but simply because it would take longer for the body to extract the protein from the vegetables than from meat or milk.
The reason we know relatively little in this area is that the potential danger of cancer promotion from glutamine availability has not been a focus of research. (On "promotion," see "Why You Should Worry about Cancer Promotion by Diet as Much as You Worry about Cancer Initiation by Carcinogens.") In particular, I don't know of any research yet on a counterpart to the glycemic index or the insulin index for the effect of a particular type of food on glutamine availability to cells. I would love to hear about any such research.
Why would cancer cells be metabolically handicapped? Thomas Seyfried's argument is that relying on fermentation is typically a key part of the path through which cells become cancerous. Or to put it another way, damaged OxPhos is the easiest way to disable the "limits to growth" that are switched on in normal cells. Here is how Thomas describes this disabling of the limits to growth at the end of section 15.1 "Revisiting Growth Advantage of Tumor Cells, Mutations and Evolution":
Davies and Lineweaver provided insightful views on the evolutionary origin of cancer [10]. They consider cancer as an atavistic state of multicellular life where long-suppressed ancestral cellular functions become reactivated or switched on. According to their view, cancer genetic or epigenetic mutations unlock an ancient “toolkit” of preexisting adaptations that allow cancer cells to survive in hypoxic environments. The Davies and Lineweaver evolutionary view of cancer is consistent in some ways with my hypothesis and with the views of Sonnenschein and Soto [33] and Szent-Gyorgyi [20]. Unbridled proliferation is the default state of metazoan cells. Unbridled proliferation existed during the oxygen-sparse α period of species evolution. This was also a highly reduced state where the ancient pathways of fermentation predominated in driving cell physiology. The appearance of oxygen gave rise to the oxidized state and the emergence of respiration. The emergence of respiration facilitated greater complexity in biological systems.
Respiration largely maintains the differentiated state of metazoan cells. Irreversible damage to respiration, coincident with a rise in fermentation, would unlock the toolkit of preexisting adaptations needed to survive in low oxygen environments. According to my view, protracted respiratory injury gives rise to compensatory fermentation or the atavistic condition in order to maintain cell viability.
"Atavistic" describes reversion to an ancient pattern. The ancient pattern for cells was fermentation, coupled with unbridled growth and cell division as long as the necessary nutrients were available.
Update, June 19, 2018. I noticed this article about a team doing research on the epigenetics of cancer at the University of Colorado Boulder:
Here is the most interesting paragraph:
“Many cancers make use of epigenetic gene silencing to promote their own growth. Medical scientists want to inhibit this cancer-causing process, but they first need to know exactly how it works,” said Nobel Laureate and Distinguished Professor Thomas Cech, senior author of the study. “Our new work contributes to the understanding of how the molecular machine responsible for gene silencing is recruited to its sites of action in human cells, determining which genes are turned off."
Don't miss these other posts on diet and health and on fighting obesity:
- Stop Counting Calories; It's the Clock that Counts
- Forget Calorie Counting; It's the Insulin Index, Stupid
- Obesity Is Always and Everywhere an Insulin Phenomenon
- The Problem with Processed Food
- Which Is Worse for You: Sugar or Fat?
- Using the Glycemic Index as a Supplement to the Insulin Index
- How Fasting Can Starve Cancer Cells, While Leaving Normal Cells Unharmed
- Why You Should Worry about Cancer Promotion by Diet as Much as You Worry about Cancer Initiation by Carcinogens
- Good News! Cancer Cells are Metabolically Handicapped
- Meat Is Amazingly Nutritious—But Is It Amazingly Nutritious for Cancer Cells, Too?
- The Keto Food Pyramid
- Sugar as a Slow Poison
- How Sugar Makes People Hangry
- Why a Low-Insulin-Index Diet Isn't Exactly a 'Lowcarb' Diet
- Hints for Healthy Eating from the Nurse's Health Study
- The Case Against Sugar: Stephan Guyenet vs. Gary Taubes
- The Case Against the Case Against Sugar: Seth Yoder vs. Gary Taubes
- Gary Taubes Makes His Case to Nick Gillespie: How Big Sugar and a Misguided Government Wrecked the American Diet
- A Conversation with David Brazel on Obesity Research
- Magic Bullets vs. Multifaceted Interventions for Economic Stimulus, Economic Development and Weight Loss
- Mass In/Mass Out: A Satire of Calories In/Calories Out
- Carola Binder: The Obesity Code and Economists as General Practitioners
- Carola Binder—Why You Should Get More Vitamin D: The Recommended Daily Allowance for Vitamin D Was Underestimated Due to Statistical Illiteracy
- Jason Fung: Dietary Fat is Innocent of the Charges Leveled Against It
- Faye Flam: The Taboo on Dietary Fat is Grounded More in Puritanism than Science
- Diseases of Civilization
- Katherine Ellen Foley—Candy Bar Lows: Scientists Just Found Another Worrying Link Between Sugar and Depression
- Ken Rogoff Against Sugar and Processed Food
- Kearns, Schmidt and Glantz—Sugar Industry and Coronary Heart Disease Research: A Historical Analysis of Internal Industry Documents
- Intense Dark Chocolate: A Review
- Salt Is Not the Nutritional Evil It Is Made Out to Be
- Confirmation Bias in the Interpretation of New Evidence on Salt
- Whole Milk Is Healthy; Skim Milk Less So
- Is Milk OK?
- How the Calories In/Calories Out Theory Obscures the Endogeneity of Calories In and Out to Subjective Hunger and Energy
- Putting the Perspective from Jason Fung's "The Obesity Code" into Practice
- 'Forget Calorie Counting. It's the Insulin Index, Stupid' in a Few Tweets
- Julia Belluz and Javier Zarracina: Why You'll Be Disappointed If You Are Exercising to Lose Weight, Explained with 60+ Studies (my retitling of the article this links to)
- Diana Kimball: Listening Creates Possibilities
- On Fighting Obesity
- The Heavy Non-Health Consequences of Heaviness
- Analogies Between Economic Models and the Biology of Obesity
- Debating 'Forget Calorie Counting; It's the Insulin Index, Stupid'
- Podcast: Miles Kimball Explains to Tracy Alloway and Joe Weisenthal Why Losing Weight Is Like Defeating Inflation
Also see the last section of "Five Books That Have Changed My Life" and the podcast "Miles Kimball Explains to Tracy Alloway and Joe Weisenthal Why Losing Weight Is Like Defeating Inflation." If you want to know how I got interested in diet and health and fighting obesity and a little more about my own experience with weight gain and weight loss, see my post "A Barycentric Autobiography."